What goes into the pea-sized splotch of brush-additive paste with which (most of us) start and end each day? Ellen Phiddian gets up close and personal with the ingredients on the label.
Apply knowledge of elements, compounds and chemical reactions, and learn about non-Newtonian fluids in this resource about the chemistry of toothpaste with Year 5, 8, 9 and 10 students. Download the student activity to make your own non-Newtonian fluid – slime!
Word Count: approx. 1300
If you’re following your dentist’s instructions (and lift your game if not), you deal with toothpaste twice a day.
Allowing for the likelihood that you might be a little less focused first thing in the morning, how often, while brushing your teeth, have you wondered what the paste is actually made of? Why is it foaming? Why does it flow the way it does? And why, precisely, is something that tastes like a mint lolly so good at protecting our teeth?
There’s a plethora of chemicals that can do each of these jobs, so toothpaste ingredients vary widely across brands and types. Let’s brush up on the chemicals that underlie each of the jobs that toothpaste does, and discover what makes toothpaste an everyday household chemical winner.
Ingredients:
Abrasives
Cleaning is an abrasive process. Toothpaste contains microscopic particles that scour bacteria, plaque and stains off our teeth – like the wiry side of a dishwashing sponge, but much smaller. These particles are tiny solids, designed to dislodge crud.
The most common abrasive in toothpaste is hydrated silica. Silica, or SiO2, is a mineral commonly found in quartz and sand. When hydrated, it becomes more gel-like – tough enough to clean teeth without damaging them. Silica’s commonly used as a food additive, for instance as an anti-caking agent in powdered products such as spices.
Another abrasive that can appear in toothpaste, usually at lower concentrations, is alumina – Al2O3 – which, among other things, is used in cosmetics, sunscreens and glassmaking.
Fluoride
You may have heard – or joined in on – arguments about the one-part-per-million fluoride that gets added to our tap water (on average). But toothpaste has much higher concentrations than that, being between 1,000 and 1,500 parts per million of fluoride. (Although hopefully, you ingest significantly less toothpaste than you do water.)
Also: The Science of Fireworks
Normally appearing alongside sodium as sodium fluoride (NaF), or with tin as stannous fluoride (SnF2), the fluoride’s doing the same job as it does in tapwater: preventing decay by strengthening teeth.
This “strengthening” process is called remineralisation. This is a process that happens when acids from food and bacteria slowly erode material from tooth enamel (demineralisation), while minerals like calcium and phosphates build it up again. Extra fluoride boosts the remineralisation side of things by allowing the enamel to form better crystalline molecules at the surface of the tooth.
Decontaminators
As well as physical cleaning, toothpaste can contain antibacterial agents to kill some of the microbes in your mouth. Stannous fluoride can do this job, as it has antibacterial properties; other metals like zinc are also commonly used.
Another antibacterial agent that pops up in some toothpaste is triclosan. It’s also used in some soaps, although there are calls from Australian experts to follow the US’s lead and ban it because there’s little evidence that it cleans hands better, but plenty of evidence that it contributes to antibiotic resistance.
Thickeners
Dollop a drop of toothpaste onto your toothbrush and you’ll note its sturdiness: unlike shampoo or handwash, it’s going to stay stuck in the form you dolloped it. (Healthdirect Australia recommends a “pea-sized amount” of toothpaste per brushload.)
But apply that pea of toothpaste to your teeth and it suddenly becomes a lot more fluid. Imagine brushing your teeth with an actual pea: while not the most structurally sound vegetable, it still takes a bit of chewing before you’ve smashed it to a consistency that can coat your gums. No chewing is required for toothpaste – you should encounter very little resistance as you brush.
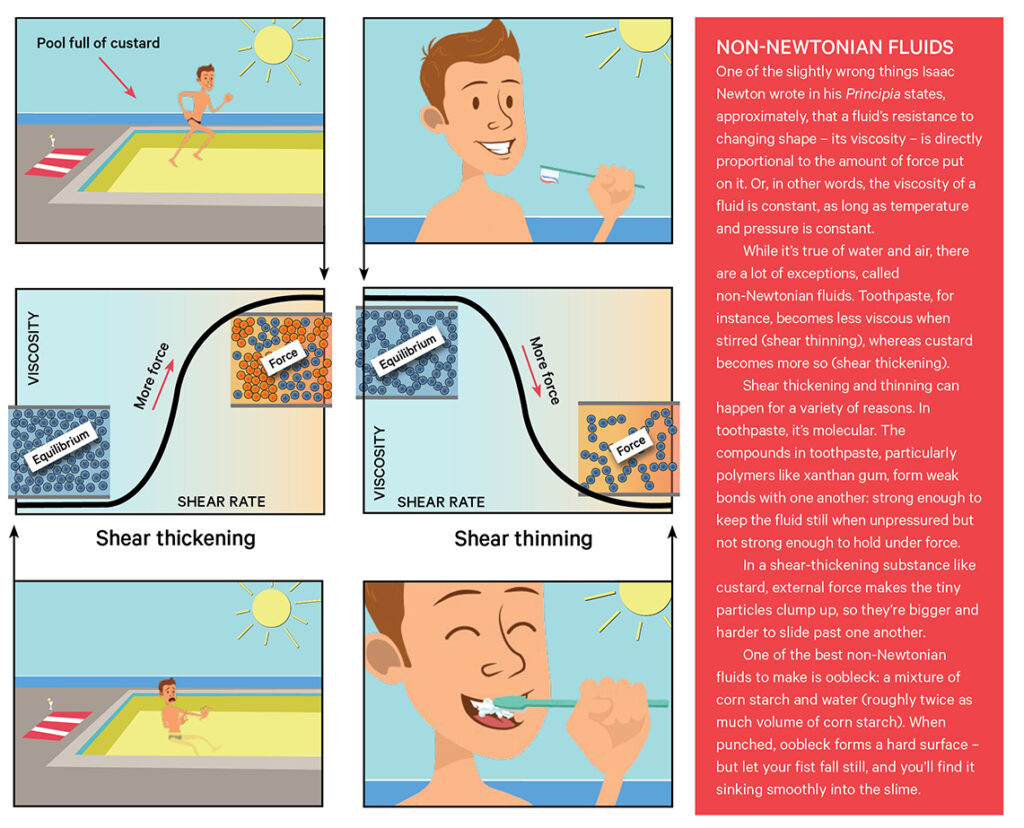
Toothpaste is a non-Newtonian fluid – its viscosity can change. Toothpaste is shear-thinning – a quality necessary for easy toothpaste use, but one that takes a lot of work and careful recipe-tuning to achieve. There is a catalogue of thickeners in toothpaste that, when paired with abrasives, give toothpaste its shear-thinning properties.
Some examples are xanthan gum (which is fermented from the bacteria Xanthomonas campestris and is commonly used as a food thickener or stabiliser), carrageenan (which is extracted from seaweed, and used as a thickener and gelling agent), and cellulose gum (or carboxymethyl cellulose, which comes from the cellulose in plants). All of these compounds are polymers: at a molecular level, they look like long chains. These chains can align neatly, forming something that resembles a solid – but a small amount of force wrecks their stability.
Companies spend a lot of time balancing their thickeners and thinners to get the perfect toothpaste viscosity. Once achieved, they also work hard to pipe the toothpaste into the tube in specific colours: while it’s sitting still in the tube, the colours won’t mix, and you can squeeze it out in delightful stripes.
Water and humectants
Water is the most abundant ingredient in toothpaste. It holds all these other ingredients together, and it’s a big part of what gives toothpaste its gel-like properties.
The substances that assist with the gelling in particular are humectants: chemicals that retain water and keep the toothpaste moist.
Glycerine and sorbitol are two of the most common humectants in toothpaste. Glycerine is commonly used in the food and medical industries – it’s a wound treatment, among other things – and in the liquid used in e-cigarettes. Sorbitol is a sugar alcohol (usually made from potato starch); it’s commonly used as a sugar substitute and a laxative. Sweet.
Surfactants
Do you notice the way toothpaste foams up as you brush, turning from a smooth gel into something filled with air bubbles? This happens because of the surfactants in the mixture.
A surfactant is something that lowers the surface tension of water, making it easier to mix with air and form bubbles. This foam is then more effective at picking up and removing grot from your teeth. Surfactants also mix well with both watery and oily substances, allowing them to clean even more effectively.
Cocamidopropyl betaine (which is derived from coconut oil mixed with a synthetic substance called dimethylaminopropylamine) and polyethylene glycols (also called PEG-6 or other numerals; derivatives of beeswax) are both common toothpaste surfactants.
Another common surfactant is sodium lauryl sulphate (SLS), which is made from a variety of oils – from petroleum to coconut. Sodium lauryl sulphate (SLS) is also the reason why orange juice tastes so terrible after brushing your teeth. SLS sits over receptors in your tongue that register sweet flavours. If you can’t taste its sweetness, the bitter and acidic flavours in orange juice rise to the fore, and the result is quite unpleasant.
SLS is a common additive in shampoo, soaps and facial cleansers, due to its foaming power. Thus, here’s an important public health message: don’t drink shampoo and orange juice together.
Colours and flavours
Silica, fluoride and xanthan gum aren’t known for their tastiness. Toothpastes have a range of colours and flavourings to make them more attractive to use – and buy. Most of these colours and flavours appear as additives in foods and drinks as well. They’re also where different brands of toothpaste differ the most: each type of toothpaste has a different cocktail of colours and flavours to set it apart.
A common ingredient you’ll see here is sodium saccharin: a very widespread artificial sweetener that does the flavouring work of sugar, without feeding bacteria like sugar does.
And one last thing…
Glance at the instructions on a tube of toothpaste and you’ll see a curious instruction for something that goes in your mouth: “Do not swallow.” Why not? Will you be poisoned if you do?
In small amounts, no. Everything is toxic if you eat enough of it, but the volume of toothpaste you use to brush your teeth is unlikely to do damage. Eat the whole tube at once, though, and some of the ingredients – especially fluoride – might reach a high enough concentration to make you a bit ill. The “do not swallow” instruction is saving you from an embarrassing call to the poison hotline, rather than thinly disguising death by dental hygiene.
This article was written by Ellen Phiddian for Cosmos Magazine Issue 93.
Cosmos magazine is Australia’s only dedicated print science publication. Subscribe here to get your quarterly fill of the best Science of Everything, from the chemistry of fireworks to cutting-edge Australian innovation.
Login or Sign up for FREE to download the educational resources