The night sky has always been a source of wonder, but it wasn’t until the 20th century that we could see the universe in its full glory – across the whole electromagnetic spectrum.
This resource is best suited to Earth and Space, Physics and Chemistry students in Years 8, 9, and 10 who are learning about elements, waves and energy. It is also suitable for senior Physics students who are learning about electromagnetic waves.
Word Count: 700
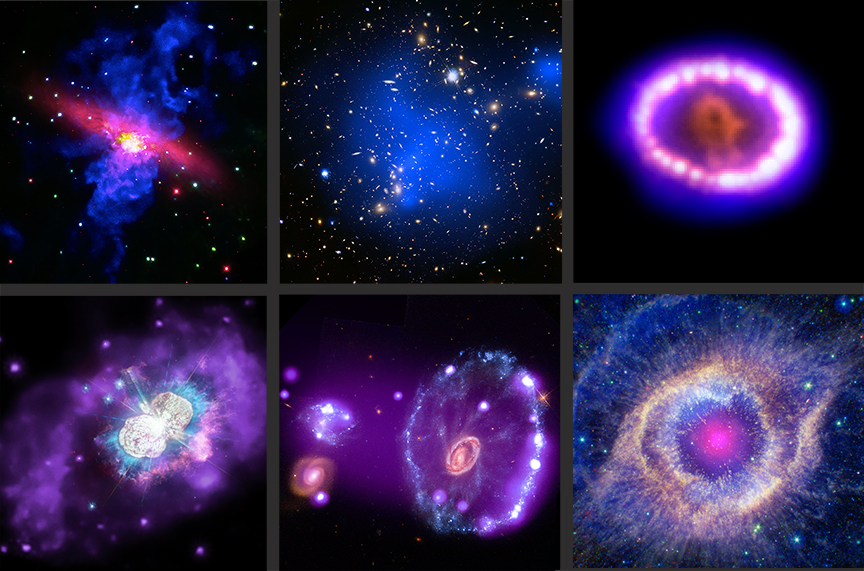
What is the electromagnetic spectrum?
The human eye has evolved to detect a specific range of colours, familiar to us as the rainbow – red, orange, yellow, green, blue, indigo and violet. Each colour corresponds to a wavelength of light: red has the longest wavelength, spanning through to violet with the shortest. But the spectrum of colours that we can see only makes up a tiny fraction of the total light in the universe.
Below red wavelengths, we find infrared light, microwaves and radio waves, while above violet we find ultraviolet light, X-rays and gamma rays. This range of light is known as electromagnetic radiation, since light is really a wave of alternating electric and magnetic energy. The entire range of light that exists is collectively referred to as the electromagnetic (EM) spectrum.
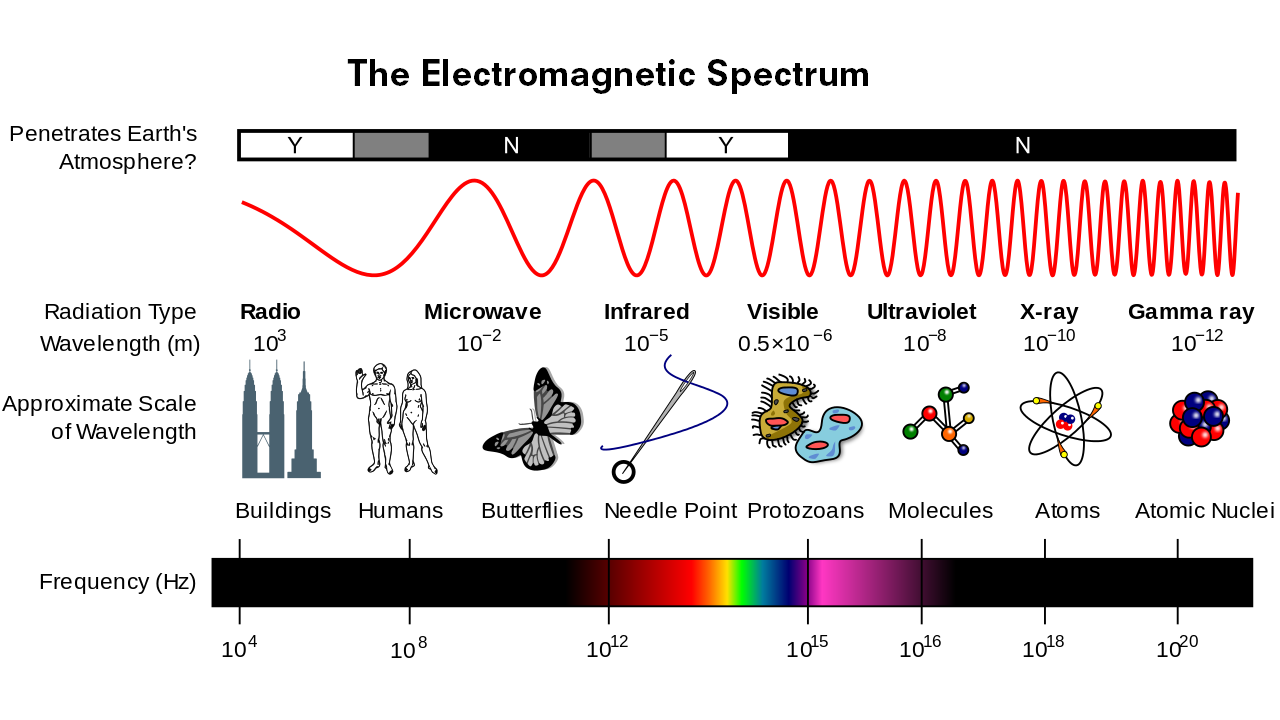
The wavelength of light is linked to its energy. Lower-energy types of light like radio waves surround us constantly and are not harmful to humans, but high-energy light like ultraviolet rays and X-rays can be dangerous – which is why we wear sunscreen on sunny days and why we’re protected by lead shields when getting an X-ray.
How can we detect the rest of the EM spectrum?
Most of the spectrum is invisible to us, though other animals can see outside of our narrow range – certain fish, bullfrogs and snakes can see infrared radiation, while butterflies, bees and some birds can see UV light.
Humans have developed a range of technologies to extend our vision and “see” other forms of light. Night-vision goggles, for example, allow us to detect infrared light, while X-ray machines allow us to peer through our skin to our bones.
Astronomers are particularly keen to see the universe across the whole EM spectrum, because celestial objects emit light at a variety of different wavelengths, often simultaneously. Telescopes have been designed to make observations from radio waves to gamma rays, uncovering information that would otherwise remain hidden if we only used optical (visible light) observations.
What can light tell us about astronomical objects?
Different types of celestial objects can be viewed using different wavelengths of EM radiation. For example, telescopes like the Square Kilometre Array – a huge array of dishes being built in Australia and South Africa – will use radio waves to look at cooler objects, studying events like the ignition of the first stars and galaxies right after the Big Bang. Near the other end of the spectrum, telescopes like the Chandra X-ray Observatory can see the bright blasts of X-rays, emitted by objects like neutron stars and black holes, superheated to tens of millions of degrees.
By combining data from a variety of telescopes spanning radio waves and microwaves up to gamma rays, astronomers can build a complete picture of the universe. This is called multiwavelength astronomy.

Unlocking the secrets of starlight
Studying the range of light emitted by an astronomical object can also give us an insight into what it’s made from. This technique, called spectroscopy, is a fundamental tool of astronomy. It involves capturing the light from an object and separating it out according to wavelength, much like a prism separates out colours, creating a “spectrum” of the object.
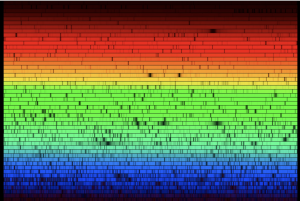
Across a spectrum, astronomers look for dark gaps called “spectral lines” that indicate missing wavelengths of light. Each of these lines corresponds to a specific element, because an atom of a certain element will both emit and absorb a specific wavelength of light. When an atom within a star or other object is hit with a photon of light, the photon is absorbed and then re-emitted it in a different direction. This means it doesn’t reach us here on Earth, creating a gap – a spectral line – on the spectrum. Each spectral line therefore corresponds to the unique fingerprint of an element, allowing astronomers to determine the chemical composition of an object.
Though first used to study stars, spectroscopy can also give us insight into many more exotic objects, such as neutron stars, black holes or active galaxies, determining what they’re made of, how fast they’re moving, their temperature and their density. Spectroscopy can even allow us to peer into the atmospheres of far-off planets around other stars.
Written by Lauren Fuge, science journalist at The Royal Institution of Australia.
Login or Sign up for FREE to download a copy of the full teacher resource