Atomic-force microscopy allows new understanding of Saturn’s moon.
This article looks at how researchers are using technology to understand the chemical make-up of Titan. It is best suited to Year 8, 9 and 10 Chemistry students who are learning about elements, compounds and molecules.
Word Count: 673

New research from the Astrophysical Journal has identified some of the intricate molecules and reactions happening on the surface of Titan, Saturn’s largest moon. In this explainer, you’ll find out how it’s done, why it’s useful and what’s next.
How do we identify individual chemicals?
Even on Earth, it can be very difficult to figure out the chemical composition of substances.
Molecules are too small to see with most technology, so chemists have to use a lot of other techniques to figure out their shapes and how their atoms are arranged.
These techniques can include things like spectrometry, which weighs atoms, and spectroscopy, which shines different types of light through molecules and examines the way it’s absorbed.
This is how many of the simple gases on solar system bodies have been identified. But the more complicated the molecule, the harder it is to determine exactly what it is.
What did we know about Titan?
We’ve known for decades that there are a lot of complicated molecules on Titan. These molecules exist in a haze above the moon’s surface, and a lot of them are organic – meaning they contain carbon and hydrogen-bonded with each other in complex structures. It’s thought that 2.8 billion years ago, Earth’s surface looked a little like Titan’s does now. So studying Titan can give some indication of what Earth was like when life was beginning to form.
More: A short history of atoms
The 1997–2017 Cassini-Huygens mission, which landed a probe on Titan in 2005 and performed several fly-bys, was able to send back a lot more information about the moon. But a spacecraft can only fit so many instruments. The mission was able to send back mass spectrometry data, which can show which elements are present and their abundance, and infra-red spectroscopy data, which yields a little information about how the atoms are bonded together. That still leaves a lot of guesswork on exactly which molecules might be present.
How could we find out more?
One trick for learning more is to recreate Titan’s haze on Earth. A stainless steel container is filled with the elements that are known to exist on Titan, in the right amounts, and conditions like temperature, pressure and light are changed to mirror Titan. The atoms inside should then start reacting and bonding like they’re on Titan. Then, instead of going to space to study the molecules, they can be examined more closely on Earth.
This technique has been used for decades to study both Titan’s haze, and that of other moons and early Earth. Some researchers used it recently to get even more detail on Titan’s haze.
What did this new research do differently?
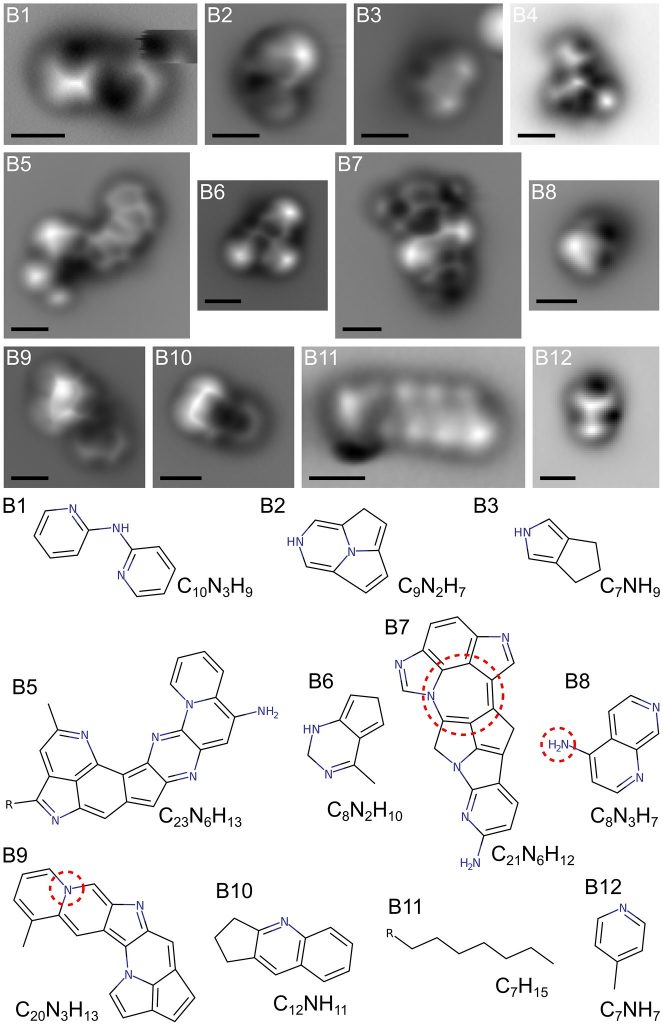
This research used atomic-force microscopy, a relatively new technology that can create images of individual molecules, to take a look at the more complicated molecules created by their Titan haze replication. The atomic-force microscopy was able to obtain pictures of around 12% of the large, complicated molecules that were in the haze.
They found a lot of the molecules were mostly linked rings of carbon, hydrogen and nitrogen, in detailed lace-like structures (pictured above). It had been theorised that these types of molecules existed on Titan before, but this is the first confirmation.
How could this be used elsewhere?
Analysing chemicals is a difficult science, but deeply important – people need to know what molecules are before they can study them further. This technique could be used to study other extra-terrestrial surfaces modelled in a lab.
“For this first time here we see the molecular architecture of synthetic compounds similar to those thought to cause the orange haze of Titan’s atmosphere,” says Conor A Nixon, a research space scientist at NASA’s Goddard Space Flight Center.
Nixon thinks atomic force microscopy will be “an exciting new tool for sample analysis of astrobiological materials, including meteorites and returned samples from planetary bodies”.
Finally, living creatures are formed of complex organic molecules. Studying these molecules on Titan can help further our understanding of how life formed on Earth – and, perhaps, how it might form on other worlds.
This article is republished from Cosmos. Read the original article.
Login or Sign up for FREE to download a copy of the full teacher resource