Three-phase Leidenfrost effect opens new understanding of heat transfer.
This resource is best suited to Year 8 and 10 Physics students looking for applications of learning methods for describing energy transfers and transformations. Also Year 8 and 10 Chemistry students looking to explore changes in matter at a particle level, and distinguish between chemical and physical change. A short video is included to help with explanations.
Word count / Video Length: 327 / 00:32 mins / 03:59 mins

If you’ve ever dropped water onto a hot pan and watched it whizz around (and if you haven’t, we can thoroughly recommend this pre-dinner experiment), you’ve observed the Leidenfrost Effect. The water boils before hitting the hot surface, making a small unstable cushion of gas that propels the liquid about.
Drop a block of ice into the pan, and you’ll just get a disappointing wet plunk. But new research in Physical Review Fluids has figured out how to achieve the Leidenfrost effect with ice, too.
“There are so many papers out there about levitating liquid, we wanted to ask the question about levitating ice,” says co-author Associate Professor Jonathan Boreyko, a researcher at Virginia Tech University, US.
“It started as a curiosity project. What drove our research was the question of whether or not it was possible to have a three-phase Leidenfrost effect with solid, liquid, and vapour.”
One of Boreyko’s then-undergraduate students, Daniel Cusumano, spent some time figuring out if there was a temperature that would make ice boil immediately. He discovered that while liquid water would levitate on an aluminium surface heated to 150°C, it took up to 550°C before the ice would do the same.
Graduate student Mojtaba Edalatpour developed a model of heat transfer, to find out why this was happening.
A slow-motion video of ice melting can be seen here.
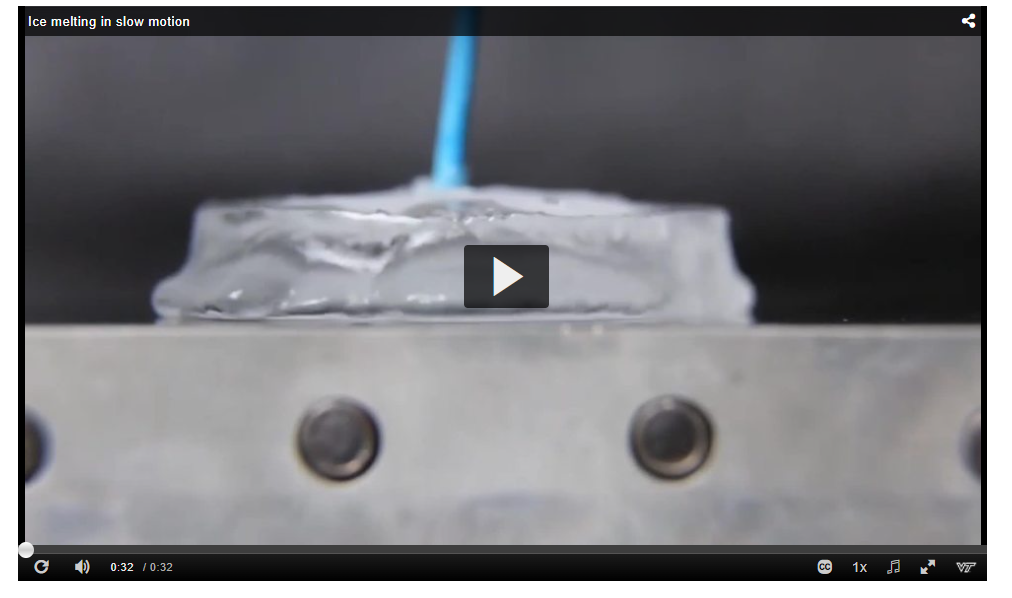
“The temperature differential the ice is uniquely creating across the water layer has changed what happens in the water itself, because now most of the heat from the hot plate has to go across the water to maintain that extreme differential,” explains Boreyko.
“So only a tiny fraction of the energy [is left to be able] to produce vapour.”
The researchers say that their modelling could be used to manipulate heat transfer in a range of places – from heating buildings and cooling computer surfaces to using ice to quench fires.
Watch the video below for an explanation of how the Leidenfrost effect occurs.
Login or Sign up for FREE to download a copy of the full teacher resource