In complete defiance of one of nature’s most fundamental laws, a species of tiny, frenetic bat is living 10 times longer – and healthier – than it should. Andrew Bain talks to the research team embarking on the project of a lifetime to find out how they do it – and whether we might do it too.
This article explores special cells of bats that allow them to live much longer than other mammals of their size. It demonstrates the importance of scientific understanding of animal biology to develop technology or solutions for humans. The article covers Biology outcomes for years 2, 3, 5, 7, and 8, however, it may need to be summarised for students in primary science.
Word Count: 2621
In the tall church towers of Brittany in northwest France, the fountain of youth stirs to life each night. In scenes that might be cut from a Gothic novel, colonies of small Myotis myotis (greater mouse-eared bats) awaken from their roosts, flying out in waves into the Atlantic darkness.
Awaiting them each July for the past seven years has been an international team of scientists and volunteers, who catch them, take a tiny sample of blood and a wing punch of tissue, then release them again into their extraordinary lives. Almost unfailingly, the same individual bats will be seen again in 12 months, and likely for many years to come, because these animals have naturally mastered one of the coveted mysteries of existence: living very long and remarkably healthy lives.
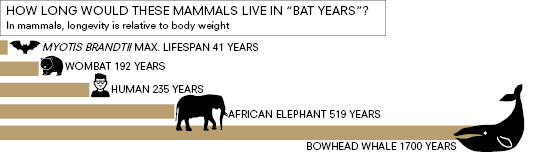
Of the 19 species of mammals that live longer than humans relative to their body size, 18 are bats, including M. myotis. The other is a naked mole rat.
“There’s a rule in nature: small things live fast and die young, and big things live slow and live long,” says Emma Teeling, the University College Dublin professor and zoologist who heads the Brittany study. “Bats are some of the smallest of all mammals, yet they can live way longer than expected given their body size.”
M. myotis typically weighs around 25 grams, which is about the same weight as a laboratory mouse. But while a lab mouse will live for no more than four years, these incredible bats can live up to 37 years. In Siberia, a Myotis brandtii (Brandt’s bat) weighing just seven grams was caught and banded in 1964. It was captured again, alive and well, almost 42 years later – a timeframe that would represent around 235 years in a human. Most remarkably, the bat showed no signs of ageing.
Contrast this to the tale of human life. The World Health Organisation has predicted a 350% increase in the number of people aged over 80 by 2050, and a doubling of the number of people aged over 60, but with no equivalent increase in the health of the population. There could be 300 million more octogenarians, nonagenarians and centenarians on the planet in 30 years, but they will be no healthier than the elderly of preceding generations.
“So everyone is going to live longer, but wouldn’t it be much better if those people were also healthy?” Teeling asks. “They’ve argued in the US that if you can slow down the ageing process by two years over a 50-year time period, it’s going to save $7 trillion. It’s about matching health-span to lifespan.
“I think that if we can find novel solutions to these problems, let’s do it, but we have to look outside the box, which means looking outside humans. You have to look at animals that have naturally evolved these mechanisms.”
And what better examples, she says, than those wise elders of the mammal kingdom: the long-lived Myotis bats.
Bats over Brittany
Though the remarkable longevity and health spans of bats of the Myotis genus were already well recorded, the mechanisms they’d evolved to allow them to slow down the ageing process weren’t well understood. These bats didn’t age, and they didn’t get cancer, but how did they achieve these superpowers of health?
Teeling saw an opportunity to try and understand and explain this mystery when a French postdoctoral student in her Dublin laboratory, Sebastian Puechmaille, mentioned a group of “crazy mad bat men” in Brittany who had been studying a population of M. myotis for the past 20 years.
Counts had been conducted, cameras set up in the attics of schools to monitor the bats, and the animals had been banded in 2010. With bats almost impossible to maintain and study in a laboratory, here suddenly was a wild population ready-made to be researched.
“They’d been studying them simply because the French love their wildlife,” Teeling says. “If the bats hadn’t been tagged for that long, we couldn’t have done what we did.”
What was really extraordinary was that as bats increased in age they would increase the expression of certain genes that we know can extend the life of model organisms.
Working each year from rented cottages around Brittany’s Morbihan department, with up to 20 researchers and local volunteers sleeping on the floor, the team would collect blood samples and biopsies through the night from a bat population spread across three Gothic churches, a school and a local’s home. The samples would be flash frozen, and the team would sleep briefly after breakfast (aided by glasses of breakfast rosé) before waking to filter the samples.
“It’s five-star field work, but after doing that for three weeks you’re very tired and you become what I call bat-lagged,” Teeling says. But with blood samples and tissue taken from hundreds of bats, the first longitudinal study of its kind has yielded fascinating results.
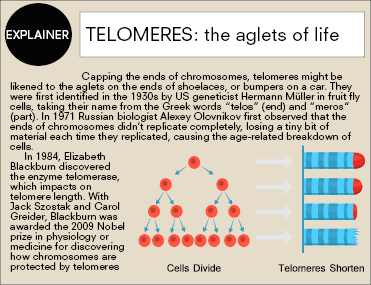
M. myotis appears to have developed a range of different life-extension methods. One of the most striking is its ability to maintain the length of its telomeres, the caps on the ends of chromosomes that prevent the chromosomes from fusing together and protect them from damage and deterioration. In humans and other mammals, telomeres shorten with age, causing a breakdown of cells that can drive tissue deterioration.
“Eventually the telomeres get so short that the cell becomes old, so either it self-destructs, which it should do, or it sits there and stops replicating – it becomes senescent,” Teeling says. “Senescent cells basically send out a call to other cells to come to them, and they become these zombie cells that can’t self-destruct, so they start emitting SASP [senescence- associated secretory phenotype], making all the other cells around them old.”
Telomeres in M. myotis, however, show no shortening, so their chromosomes remain protected throughout their lives. Unlike cancer or germline cells, which are the only human cells on which telomeres don’t shorten, this isn’t achieved through the expression of the telomarese enzyme.
Over six years, Teeling and her team looked at around 400 different genes, identifying two – SETX and ATM – that were potentially driving this process. The same genes are also involved in repairing DNA damage. “These genes seem to be evolving differently in bats than other mammals, and maybe this underlies their ability to maintain their telomeres,” she says. “We’re going back in the lab now, developing bat cell lines to try to see if these genes are doing what we say they’re doing.”
More: To get big, grow slow
Another secret to the bats’ eternal youth rests in their mitochondria, the organelles that are the so-called powerhouses of cells. Gifted with the energetic and demanding power of flight, bats have incredibly high metabolic rates – during flight there can be a 15-fold increase in their oxygen consumption. Such a workload should, by rights, shorten a bat’s life, but instead these animals are retaining all the energy of youth into their defiant old age. It’s the natural order turned on its echo-locating head.
Teeling’s team of researchers found that the bats were producing high levels of free radicals, but they weren’t displaying the damage to their mitochondria that this would typically result in. “We had this ridiculously high metabolic rate without the expected damage,” she says. “This means bats are finding ways to either repair the damage or remove the damage.”
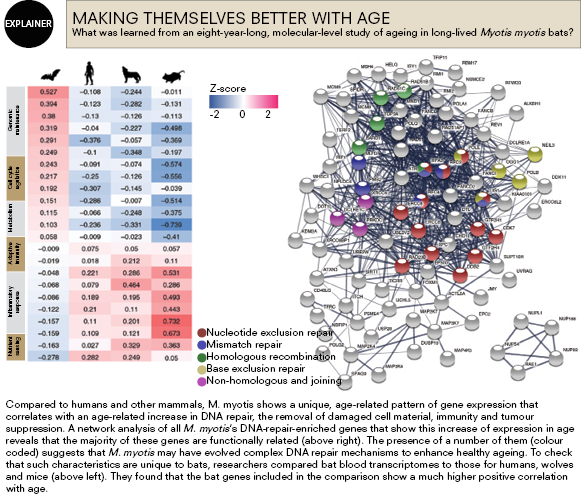
One of the study’s most challenging tasks was deep sequencing around 1.7 trillion base pairs of RNA from blood samples taken from 70 individual bats to try to uncover what was happening at the molecular level to prolong their health and life. Researchers attempted to sequence the entire blood transcriptome – the set of all RNA molecules – using as little as 40 microlitres of blood, or fewer than two drops, taken from bats across a range of ages.
Comparing the transcriptome to data sets for humans, mice and wolves – the only other data sets available – the team found that while the body’s ability to repair DNA decreases with age in the other mammals, it actually increases with age in long-lived bats. Similarly, while humans get high levels of inflammation as we age, Myotis bats don’t show this – they remain proficient at dampening inflammation even into their later years.
“What we found that was really extraordinary was that as bats increased in age they would, for example, increase the expression of certain genes that we know can extend the life of model organisms,” Teeling says.
“For example, if you stick an extra copy of this thing called a PTEN gene into a lab mouse, it will live longer, or if you cause it to downregulate its expression of [protein-coding gene] MYC, it lives longer. Bats naturally do this. What that meant was that this crazy idea that we came up with works – we can uncover signatures of longevity.”
Quite simply, these long-lived bats don’t seem to experience age-related mortality. They die instead from the likes of fungal infections, starvation or accidents. “I don’t think it’s too broad-sweeping to say they’ve found the fountain of youth,” Teeling says.
Just Add Humans
The robust eternal youth of bats is an extraordinary piece of evolutionary excellence in itself, and fitting for a mammal already equipped with other evolutionary talents as impressive as flight and echolocation.
But the true value of the study of M. myotis will most likely come in its future application for humans. University of NSW zoologist Professor Sue Hand whose special interest is in fossil and modern bats, particularly admires Teeling’s work. “I think the work of that group is very elegant, and it answered a lot of questions,” she says.
“But I think it’s also opened up a whole lot of other avenues for exploring. The thought that this information might be able to extend the liveable part of human life, keeping people healthier for longer, is really exciting.”
For Teeling, the long-lived bats seemed an obvious model for exploring the possibilities of extending human life and, more importantly, the duration of our good health.
Most studies on ageing have focussed on model organisms – the mice and nematodes of laboratory work – vastly expanding our understanding of the ageing process. Teeling believes, however, that model organisms have only so much to offer in further broadening our knowledge of ageing mechanisms. Model organisms typically live fast and die young – they’re great at dying, but not so good at living – so why not take a new approach and study the masters of healthy ageing?
In its study of M. myotis, Teeling’s research team found new genes that hadn’t been previously identified, but they also discovered a host of new microRNAs, which regulate the genes, causing them to switch on and off – controlling the bats’ antiageing signatures. Teeling describes the microRNAs as akin to master switches and suggests they may hold the key to translating the bats’ powers of longevity, including their resistance to cancer, into humans. In ageing bats, for example, microRNAs that act to suppress tumours are upregulated, while those promoting tumours are downregulated.
“What we found was a whole bunch of these microRNAs that we think are regulating the longevity signature, and that’s what we need to work on now to prove – can they somehow extend life?” Teeling says.
“In the next study we’ll be validating that they are microRNAs, that they do what they should be doing, and then finding out what their targets are. And then we need to see if we can find the equivalent in humans.
“By finding the microRNAs it’s like we’ve found the master switch, and that’s where you can make a microRNA drug [for humans] to do what we saw the bats were doing.”
A vast journey lies between this detection in bats and any future application in humans, but Teeling is in no doubt about the biomedical value of the study’s findings. “I do believe in the translation,” she says. “We have exactly the same sort of genes – it’s just how we turn them on or turn them off, or when we turn them on or off. As you can see from COVID-19, we’re just one other species amongst everything. Viruses or zoonotics can hop between species, so why can’t we also look at the genes that species have that can be to our benefit?”
Others in the field agree. “A lot of the different things Emma’s group looked at in the study, people had gone along those tracks before,” says Hand. “But I think the work they did actually brought a lot of those elements together. Some of these could certainly be used to see how we might be able to tinker with the human system.”
Bat1K
One tangible aside of the M. myotis study is Bat1K, a consortium Teeling founded in 2018 with Sonja Vernes of the Max Planck Institute for Psycholinguistics in the Netherlands. Similar to concurrent projects such as Genome10K, the Earth BioGenome Project and Oz Mammals Genomics, Bat1K has the ambitious goal of sequencing the genomes of the world’s 1400 bat species.
So far it has brought together more than 400 people from across the globe dedicated to this idea of sequencing the second-largest mammal group on earth. To date, the genomes of six bat species have been sequenced, leaving a monumental task still ahead.
“It’s huge,” says Professor Katherine Belov, a geneticist and pro vice chancellor of global engagement at the University of Sydney who is currently in discussion with Teeling to collaborate on the project.
“But sequencing is becoming cheaper, and our main stumbling blocks now are getting the samples and getting good enough quality samples. I think the whole world is on this journey of uncovering the genetic library of life on Earth, and I think the bats will be particularly fascinating.”
At its core, Bat1K is about producing what Teeling describes as an “exquisite, excellent, near-complete, near-perfect genome of every living bat”, of the sort that exist for humans and model organisms. It will capture the diversity of these creatures that make up around 20% of all the living mammals on the planet, and create the tools researchers will need to continue studying bats’ unique adaptations.
We found these microRNAs that we think are regulating the longevity signature, an that’s what we need to work on now – can they somehow extend life?
“Most of the genomes from things that aren’t model organisms or domestic species are fragmented and difficult to work with,” Teeling says. “Because it’s become cheaper to be able to build beautiful genomes, we’ve been doing that for Bat1K. These are the genomes that people are going to need to use to try to understand, for example, where COVID-19 comes from and how we can better tolerate it.”
As the Bat1K project rolls out, the Brittany study of M. myotis rolls on. The first microchipped bats are now around 10 years of age – mere youths, given their life expectancy – and Teeling hopes to observe them for the duration of their lives. Funding permitting, it will mean returning to Brittany every July for around 25 more years, providing a stunningly complete picture of the bats’ lives.
Coupled to this are plans to study shorter-lived bats, comparing and contrasting their genetics against those of M. myotis in the hope of identifying the particular genes that might be extending the lives of these durable flappers, and truly unravelling their secret to a long and healthy life.
“My ultimate goal is that some of my research might end up alleviating people’s suffering,” Teeling says. “I think that by studying bats we might eventually come up with a pill or some other idea, but I just want to find the answer that can then be taken to the next level.
“This is the project of a lifetime, and I think it’s going to take an awful lot of time. I don’t know if I’m going to live long enough to do it, but maybe from the ideas I’ve come up with, other people will.”
This article was written by Andrew Bain, Cosmos contributor based in Hobart, for Cosmos Magazine Issue 87.
Cosmos magazine is Australia’s only dedicated print science publication. Subscribe here to get your quarterly fill of the best Science of Everything, from the chemistry of fireworks to cutting-edge Australian innovation.
Login or Sign up for FREE to download the educational resources