UniSA researchers refine solar evaporation for clean water tech.
This resource shows how scientists are using their understanding of Chemistry, Earth Science and Physics to design technology to help society. It is best suited to students in Years 5, 7, 8 and 10 who are learning about heat energy, evaporation and condensation and the water cycle.
Word Count: 575

Researchers from the University of South Australia (UniSA) are currently developing and refining a low-cost technique that can derive freshwater from seawater, brackish water or contaminated water – powered by sunlight.
Freshwater scarcity is one of the most serious global challenges in the face of a growing population, changing climate and pollution. Currently, 1.42 billion people live in areas of high or extremely high water vulnerability, and so in recent years, research has been focused on developing new materials and technologies to turn seawater or polluted water into safe, drinkable water.
Australia, for example, currently has six major desalination plants providing freshwater to Perth, Adelaide, Sydney, Melbourne and southeast Queensland. These plants largely rely on reverse osmosis, which uses a membrane to separate salt from water.
It relies on squeezing water through incredibly tiny pores that salt can’t pass through, which takes a lot of energy because it breaks the chemical bonds between the water and salt molecules.
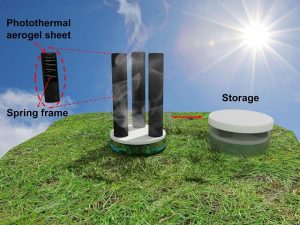
But the technology being developed by Haolan Xu and colleagues at UniSA works in a different way, instead harnessing the Sun’s energy to clean water.
“In recent years, there has been a lot of attention on using solar evaporation to create fresh drinking water, but previous techniques have been too inefficient to be practically useful,” says Xu.
“Our technology can now deliver enough fresh water to support many practical needs at a fraction of the cost of existing technologies like reverse osmosis.”
The system works using a highly efficient photothermal structure, which converts sunlight into heat and concentrates it on the surface of water to evaporate the upper part of the liquid. While other research into similar technology has been plagued by energy loss, losing 10 to 20% of solar energy, this technique bypasses that problem by using a 3D structure.
This, Xu explains, “not only increases the overall evaporation surface area, but also introduces cold evaporation surfaces (i.e. the surface without solar light irradiation) which can harvest additional energy (other than solar light) from the surrounding air and bulk water to enhance overall water evaporation”.
When there is strong sunlight, the system is able to deliver between 10 to 20 litres of freshwater per square metre of source water per day.
It is also built from simple, everyday materials.
“One of the major raw materials such as substrate material (e.g. cotton sheet) can be purchased from local market such as Woolworths,” Xu says.
Its size is also very portable: “The unit evaporator can be tens of centimetres to one metre in lateral size. For large scale use, it can be realised by simply arranging and connecting a number of units.”
The system could therefore be used to treat wastewater in industrial operations, or accelerate water evaporation in mining industries (such as in tailing ponds or dams).
But since the system is simple, cost-effective and needs almost no maintenance, it would also be useful to provide people and communities with low-cost, long-term clean water solutions.
“For instance, in remote communities with small populations, the infrastructure cost of systems like reverse osmosis is simply too great to ever justify, but our technique could deliver a very low-cost alternative that would be easy to set up and basically free to run,” Xu says.
So just how long could this take?
“I think it can be commercialised for practical use in the following a couple of years,” he concludes.
The team’s most recent paper is published in the journal Solar RRL.
This article is republished from Cosmos. Read the original article.
Login or Sign up for FREE to download a copy of the full teacher resource